- Home
- MCQs
- Cases
- Flashcards
- Your Feedback
- Become a reviewer
- More student books
- Student Apps
- Join an e-mail list

You see two patients in casualty. Jill is a 40-year-old office worker with diabetes who is away from home but forgot to bring her insulin; she is now hyperglycaemic, and her breathing is heavy and laborious. Sheila is a 20–year–old student who attempted suicide by taking an aspirin overdose last night. Their ABG and blood analyses are shown below.
| Jill | Sheila | Reference values | |
|---|---|---|---|
| pH | 7.25 | 7.56 | 7.35–7.45 |
| PaCO2 (kPa) | 3.1 | 2.9 | 4.7–6.1 |
| PaO2 (kPa) | 10 | 12 | 12.2–13.9 |
| [HCO3–] (mmol/L) | 14 | AD | 22–26 |
| [K+] (mmol/L) | 3.7 | 4.5 | 3.5–5.5 |
| [Na+] (mmol/L) | 135 | 137 | 135–145 |
| [Cl-] (mmol/L) | 95 | 99 | 96–106 |
1. What is your diagnosis of the acid–base status of these two patients, and what is the cause their acid–base disorders?
2. How might Sheila’s acid–base status have differed if you had seen her shortly after she ingested the aspirin?
The history and preliminary evaluation of the patient may provide important clues as to the disorder and underlying problem, for example, respiratory dysfunction, vomiting, diarrhoea, diabetes.
| Step 1 | Are ABG values consistent and thus valid? | ||||||||||||
| Step 2 | Is there an acidaemia or alkalaemia?
|
||||||||||||
| Step 3 | Is the (primary) disorder respiratory or metabolic?
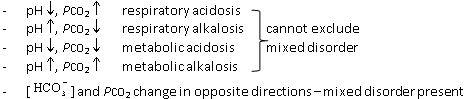 |
||||||||||||
| Step 4 | Is compensation appropriate for primary disorder?
These empirical rules (based on experience) give an estimate of what [HCO3–] or PCO2 would be if there was only the one disorder and there was normal compensation (N.B. PCO2 in kPa). Respiratory compensation is very rapid; renal compensation may take days to reach full effectiveness. Greater or lesser changes (outside the +/− range) suggest a mixed disorder. For use as a guide only, and confirm diagnosis from the history and further tests as appropriate.
|
||||||||||||
| Step 5 | If metabolic acidosis suggested, calculate anion gap (AG) Normally ~12 mmol/L (Chapter 11, and see Fig. 11c and 11d) Note that hypoalbuminaemia reduces AG by ~2.5 mmol/L per 10g/L reduction in plasma albumin |
3. Normal AG suggests simple loss of (hyperchloraemic acidosis, Fig. 11c and 11d).
4. High AG (>20) suggests production of metabolic acids or renal failure.
| Step 6 | If AG increased, assess relationship between changes in AG and [HCO3–] (Delta ratio)
|
| Note | This example of a step approach is based on several published guidelines. For a useful tutorial, see: http://www.anaesthesiamcq.com/AcidBaseBook/ABindex.php |